Cancer Care @ Home a Growing Trend, Proving Safe and Effective
April 5, 2021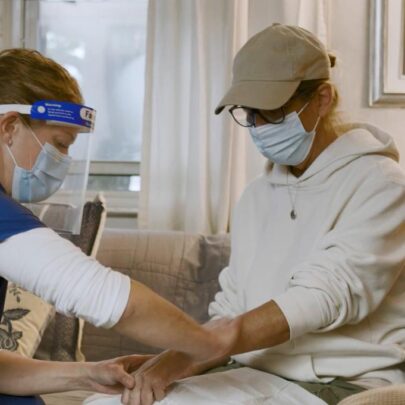
Through Cancer Care @ Home (CC@H), Penn Medicine and the Penn Center for Cancer Care Innovation (PC3I) are at the forefront of at-home cancer care in the United States. PC3I has demonstrated that many cancer therapies can be safely and effectively administered at home.
“Administering cancer drugs at home is not just feasible but is an evidence-based practice. It has been tested, it works, it’s safe, and it’s effective,” PC3I Director Justin Bekelman, MD, shares with Medscape in a new series on the growing trend.
Cancer care in the home is not a new concept, and has been embraced across Europe, Canada, and Australia for some time. For example, the United Kingdom has employed mobile cancer units since 2003, bringing care to patients particularly in rural areas. Also in the UK, the Clatterbridge Cancer Centre in Merseyside has offered at-home services for nearly 7 years, expanding over time to include a range of drugs based on guidelines published by the National Institute for Health and Care Excellence.
In spite of its success and proven safety abroad, at-home care has at times been met with skepticism and uncertainty in the United States. Some worry about safety and adverse reactions, separation between physicians and patients, feasibility, or cost.
Prior to the launch of CC@H, PC3I and partners at Penn Medicine devoted months to designing workflows and assessing which drugs could be administered safely in the home and which patients could receive treatment safely at home. The program launched in mid-February 2020, with seven drugs being administered to patients in their homes.
With the onset of COVID-19, Penn Medicine was met with a heightened urgency to move care to the home where appropriate, and the CC@H pilot quickly grew from 39 patients in mid-March to nearly 500 patients by July. Additionally, the list of drugs was expanded from seven to 13, all of which were carefully selected and administered in the home using safety protocols similar to those used in the clinic or inpatient setting.
The point is not to substitute. It is to give patients the option of receiving the treatments at home that they can and that are safe.
– Justin Bekelman, PC3I Director
In order to manage any adverse reactions that may occur, Penn Medicine’s oncology nurses are equipped with emergency kits and the same medications available in infusion suites. They survey the home environment to ensure medications can be stored properly and at the appropriate temperatures. Lindsay Zinck, MSN, RN, OCN, Associate Chief Administrative Officer for Operations for Penn Medicine’s Cancer Service Line, notes to Medscape that in the home, “[adverse] reactions are actually managed pretty similarly” to infusion suites.
In addition to safety concerns, some harbor apprehension regarding financial viability—in particular, physician-owned community practices, which already face growing expenses and declining revenues, fear losing the margins they make on drugs to home infusion providers. While Penn Medicine’s infrastructure has allowed for home infusion to become the standard of care for some treatments, like leuprolide for breast cancer and prostate cancer and EPOCH for lymphoma, independent practices may not have the infrastructure in place to bring such treatments to patients’ homes. Cognizant of the burdens faced by independent oncology practices, PC3I has shared ways to preserve community-based care in JAMA Oncology.
On the insurer side, many companies believe rates for at-home care will be lower, but Bekelman suggests that until costs associated with home infusions are better understood, it’s best for payers to reimburse at the same rates for care regardless of delivery location. “It’s not practical to start by setting the price low early on because then health systems won’t be incentivized to deliver care at home,” says Bekelman to Medscape. Additionally, Bekelman notes that insurance benefits must ensure that home infusion is a financially feasible option for patients.
Others in the United States are beginning to adopt administration of cancer treatment in the home. In June 2020 the FDA approved an injectable combination of pertuzumab and trastuzumab intended to be given at home, and in January 2021, CVS announced an agreement with Cancer Treatment Centers of America to provide at-home chemotherapy. Intermountain Healthcare in currently conducting two pilots to explore immunotherapy and chemotherapy at home. PC3I and Penn Medicine have laid a foundation for the delivery of home cancer treatment in the United States as a safe, patient-centered alternative to traditional care delivery models.
Read part one, part two, and part three of the Medscape series.